Important Information
This website is managed by Ultima Markets’ international entities, and it’s important to emphasise that they are not subject to regulation by the FCA in the UK. Therefore, you must understand that you will not have the FCA’s protection when investing through this website – for example:
- You will not be guaranteed Negative Balance Protection
- You will not be protected by FCA’s leverage restrictions
- You will not have the right to settle disputes via the Financial Ombudsman Service (FOS)
- You will not be protected by Financial Services Compensation Scheme (FSCS)
- Any monies deposited will not be afforded the protection required under the FCA Client Assets Sourcebook. The level of protection for your funds will be determined by the regulations of the relevant local regulator.
Note: Ultima Markets is currently developing a dedicated website for UK clients and expects to onboard UK clients under FCA regulations in 2026.
If you would like to proceed and visit this website, you acknowledge and confirm the following:
- 1.The website is owned by Ultima Markets’ international entities and not by Ultima Markets UK Ltd, which is regulated by the FCA.
- 2.Ultima Markets Limited, or any of the Ultima Markets international entities, are neither based in the UK nor licensed by the FCA.
- 3.You are accessing the website at your own initiative and have not been solicited by Ultima Markets Limited in any way.
- 4.Investing through this website does not grant you the protections provided by the FCA.
- 5.Should you choose to invest through this website or with any of the international Ultima Markets entities, you will be subject to the rules and regulations of the relevant international regulatory authorities, not the FCA.
Ultima Markets wants to make it clear that we are duly licensed and authorised to offer the services and financial derivative products listed on our website. Individuals accessing this website and registering a trading account do so entirely of their own volition and without prior solicitation.
By confirming your decision to proceed with entering the website, you hereby affirm that this decision was solely initiated by you, and no solicitation has been made by any Ultima Markets entity.
I confirm my intention to proceed and enter this websiteTonix Pharmaceuticals Stock Prediction 2025
Analysts forecast Tonix Pharmaceuticals (TNXP) stock to reach between $60 and $70 in 2025, reflecting a potential 30–50% upside from mid-2025 levels. The prediction is based on strong momentum, insider buying, and a key FDA decision on TNX-102 SL scheduled for August 2025. If approved, it could mark the first new fibromyalgia treatment in 15 years and drive further gains.
What Does TNXP Do?
Tonix Pharmaceuticals Holding Corp (NASDAQ: TNXP) is a clinical-stage biopharmaceutical company focused on central nervous system (CNS) disorders, rare diseases, and infectious diseases. Headquartered in Chatham, New Jersey, Tonix is developing innovative therapies including TNX-102 SL for fibromyalgia, TNX-801 for smallpox/mpox, and TNX-1500 for immune-related conditions.
As of Q1 2025, Tonix reported $2.43 million in revenue and a net loss of $16.83 million (EPS of -$2.84), beating consensus estimates. The company maintains a strong cash position of $131.7 million, expected to fund operations through mid-2026. With zero debt and strategic pipeline prioritization, Tonix is positioned for potential long-term growth.
TNXP Stock Financial Overview
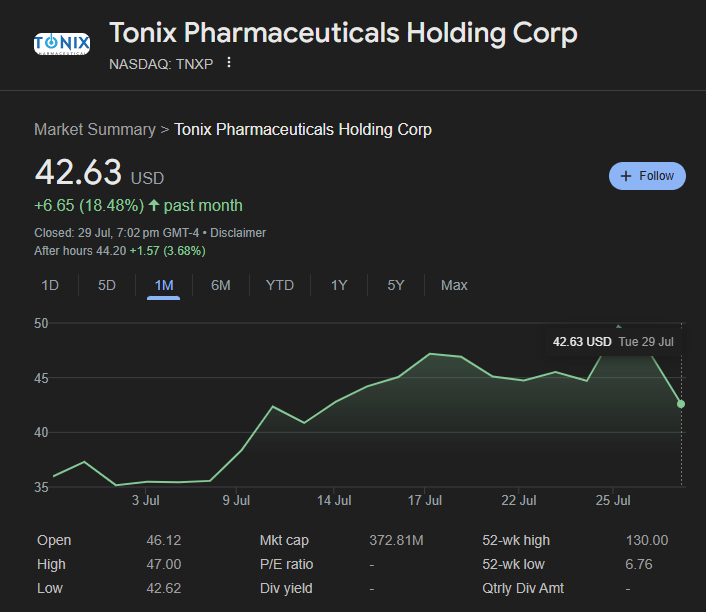
Tonix’s financial health has improved, with lower operational expenses and strategic equity raises. Despite negative earnings and a high price-to-sales ratio (~29x), its robust pipeline and insider confidence have attracted trader attention.
- Q1 2025 Revenue: $2.43 million
- Net Loss: $16.83 million (-$2.84 EPS)
- Cash Reserves: $131.7 million
- Debt: None
- Short Interest: ~16.4% of float
- Insider Buying: ~$86K by CEO in May 2025
- Institutional Ownership: ~82%
TNXP Stock Forecast 2025: Expert Analysis
Analyst Price Targets
Analyst projections for Tonix Pharmaceuticals stock in 2025 suggest moderate upside:
- Average Target: $61.67
- High Estimate: $70
- Low Estimate: $50.50
These targets reflect cautious optimism based on upcoming catalysts, especially the FDA decision on TNX-102 SL.
Technical Momentum
TNXP stock surged over 300% from May to July 2025, driven by strong earnings, insider activity, and anticipation of regulatory approval. Its Relative Strength Rating (RS) improved from 89 to 92, indicating strong market momentum. Although it hasn’t entered a confirmed technical buy zone, the stock is showing strong support above key moving averages.
The Relative Strength Index (RSI) remains elevated, nearing 70, suggesting the stock is approaching overbought territory. Volume spikes during key announcements confirm bullish sentiment, while the 50-day moving average is trending upward, reinforcing the short-term uptrend. However, traders should watch for potential pullbacks or consolidations before the FDA decision.
Fundamental Drivers
Beyond technicals, Tonix’s forecast hinges on the successful approval of TNX-102 SL for fibromyalgia. If approved by the FDA on or before the August 15, 2025 PDUFA date, it would mark the first fibromyalgia treatment in over 15 years, opening a multi-billion-dollar addressable market. Additionally, advancements in TNX-801 and TNX-1500 for infectious and autoimmune diseases could position Tonix as a long-term player in high-need therapeutic areas.
Other fundamental drivers include high institutional ownership, CEO insider buying, and the absence of debt, which provide financial stability and investor confidence. The company’s ability to consistently fund operations through mid-2026 without needing dilutive financing also supports the bullish outlook.
Will Tonix Pharmaceuticals Ever Recover?
Recovery prospects for Tonix depend heavily on clinical and regulatory milestones. The most significant near-term catalyst is the FDA’s decision on TNX-102 SL for fibromyalgia, with a PDUFA goal date set for August 15, 2025. This would be the first new fibromyalgia treatment approved in over 15 years.
Pipeline advancements in TNX-801 and TNX-1500 also support long-term recovery. TNX-801’s recent animal data show high efficacy and low virulence, and TNX-1500 is currently in Phase 1 trials for autoimmune and transplant applications.
Why is TNXP Stock Going Up?
TNXP stock is gaining momentum in 2025 due to a combination of strong clinical data, positive financial performance, and increased investor confidence.
Key Drivers:
- Positive Phase 3 Results: TNX-102 SL met clinical endpoints for fibromyalgia treatment, a major milestone.
- FDA Decision Approaching: Anticipation of FDA approval by the August 15, 2025 PDUFA date has fueled buying interest.
- Insider Confidence: CEO Seth Lederman purchased over $86,000 worth of stock, signaling insider faith in future prospects.
- Index Inclusion: TNXP was added to the Russell 2000 and 3000 indexes in June 2025, increasing visibility and attracting institutional flows.
- Institutional Ownership: 82% institutional backing reflects strong market trust.
- Technical Strength: Relative Strength Rating (RS) increased to 92, with RSI approaching overbought levels, confirming technical momentum.
The combination of regulatory milestones, technical breakout signals, and investor confidence has made TNXP one of the most watched biotech stocks in 2025.
Will Tonix Pharmaceuticals Stock Go Up?
Based on expert analysis and market trends, TNXP stock has potential to rise to the $60–$70 range in 2025 if the FDA approves TNX-102 SL and pipeline momentum continues. However, speculative models predicting extreme prices (e.g., $2,000+) are not supported by credible data.
- Bull Case: FDA approval + strong pipeline progress could push TNXP beyond $70
- Base Case: Moderate gain to $60–$65 driven by regulatory and institutional support
- Bear Case: Delays or clinical setbacks may return TNXP to $30–$40 levels
Conclusion
Tonix Pharmaceuticals stock forecast for 2025 reflects cautious optimism rooted in strong catalysts, including FDA decisions and advancing pipeline programs. With solid cash reserves, no debt, insider buying, and high institutional ownership, TNXP is well-positioned for recovery and potential upside.
Investors should closely monitor the FDA decision in August 2025 and subsequent clinical developments. While volatility remains, TNXP’s combination of regulatory milestones and technical momentum makes it a stock worth watching in the biotech sector.
For real-time updates, professional charting tools, and trader-focused insights on biotech movers like TNXP, visit Ultima Markets, your trusted partner in navigating complex markets.
Disclaimer: This content is provided for informational purposes only and does not constitute, and should not be construed as, financial, investment, or other professional advice. No statement or opinion contained here in should be considered a recommendation by Ultima Markets or the author regarding any specific investment product, strategy, or transaction. Readers are advised not to rely solely on this material when making investment decisions and should seek independent advice where appropriate.












